Bio-Efficacy of Diatomaceous Earth, Household Soaps, and Neem Oil against Spodoptera frugiperda (Lepidoptera: Noctuidae) Larvae in Benin
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Efficacy of Locally Available Soaps and Detergents against FAW Larvae
2.1.1. Experimental Conditions
2.1.2. Insect Rearing
2.1.3. Soaps and Detergent
2.1.4. Bioassay
2.2. Field Efficacy of Several Management Options against FAW
2.2.1. Experimental Sites
2.2.2. Materials Tested
2.2.3. Experimental Design
2.2.4. Data Collection
2.3. Statistical Analyses
2.4. Profitability Analysis
3. Results
3.1. Larvicidal Activity of Soap and Detergent Solutions on Second-Instar Larvae of FAW in Laboratory
3.2. Field Efficacy of Dezone, Palmida Soap, and other Management Options Evaluated
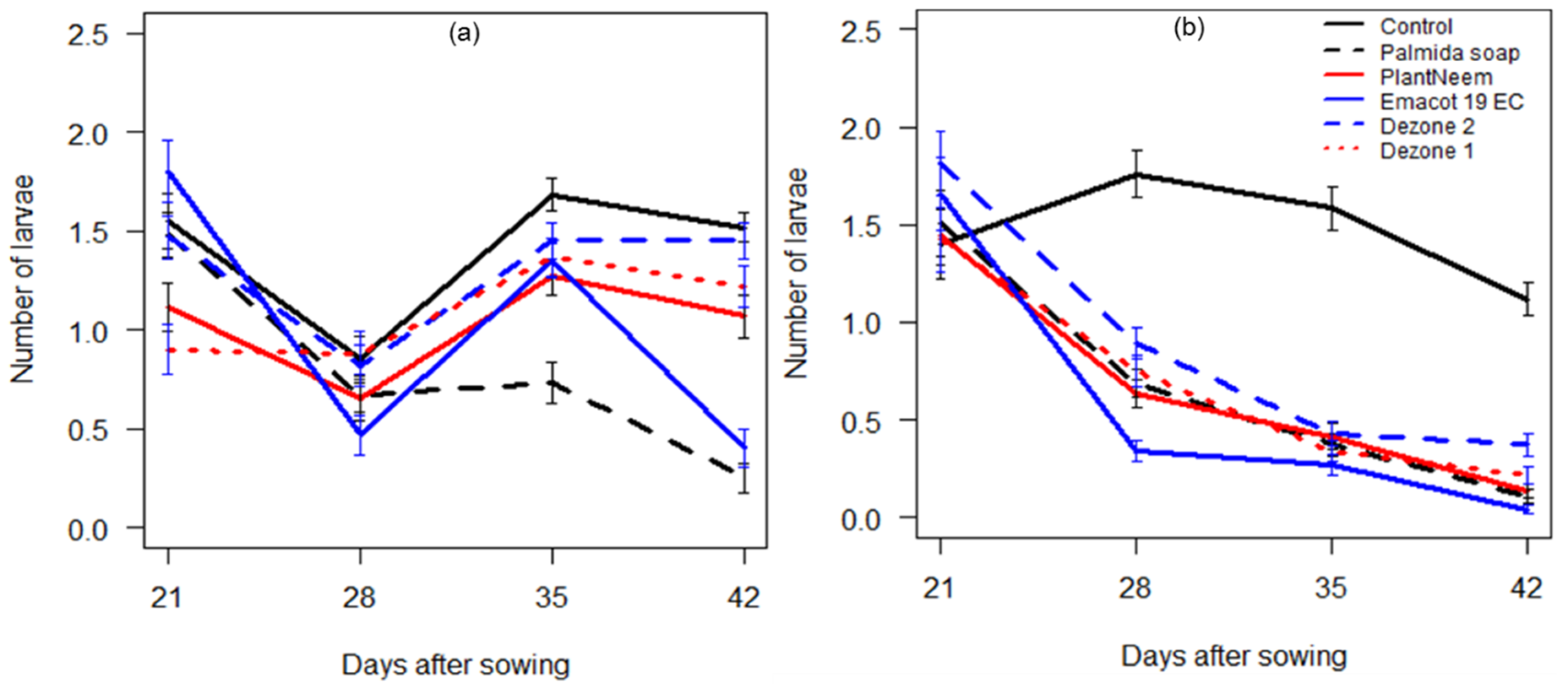
3.2.1. Impact of Insecticides Application on FAW Population
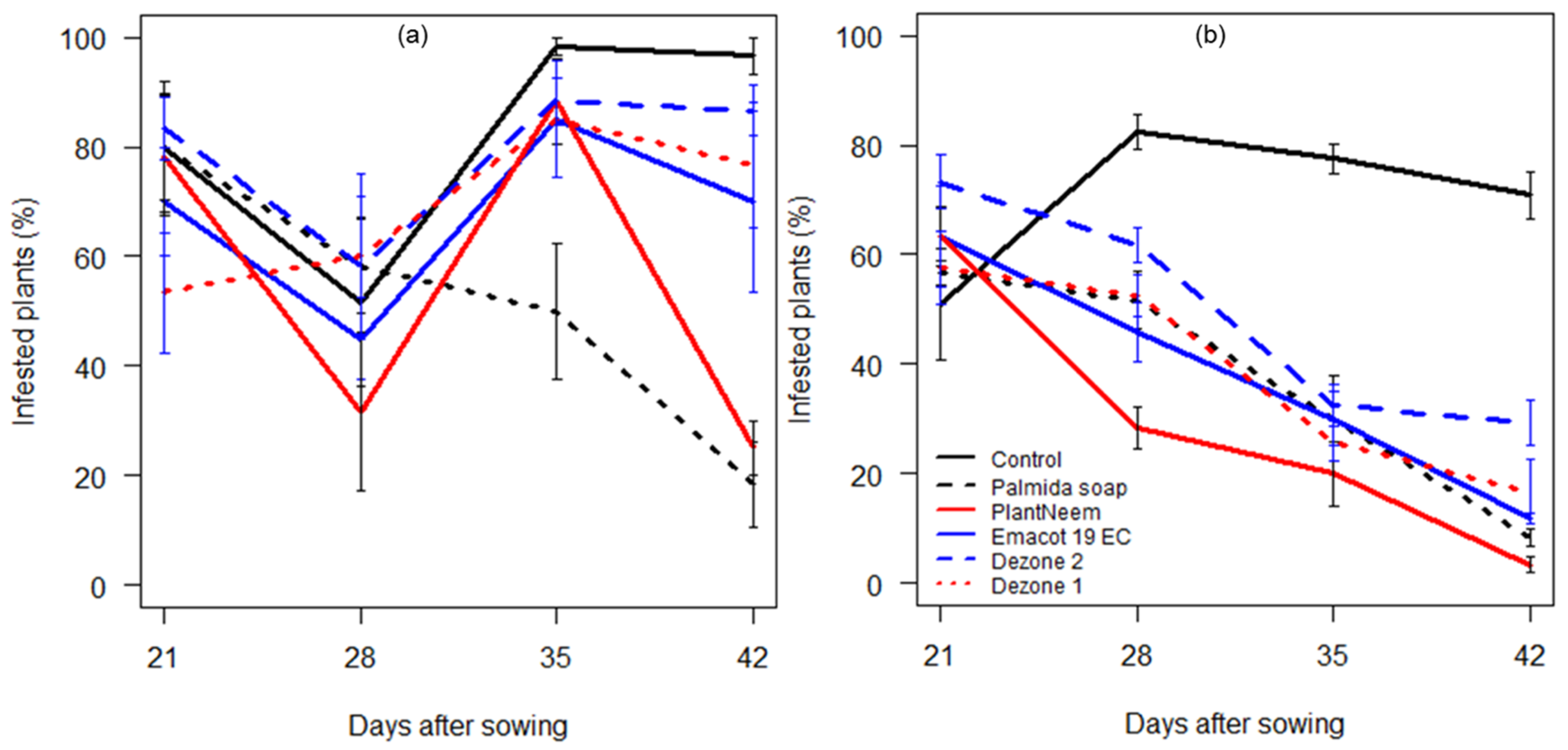
3.2.2. Prevalence of Infested Plants
3.2.3. Impact of Insecticides Application on FAW Damage
3.2.4. Phytotoxicity Level Assessment
3.2.5. Impact of Treatments on Growth Parameters
3.2.6. Yields and Percentage Reduction in Grain Yield Loss
3.2.7. Cost-Benefit Comparison Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamo, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J.E. Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [PubMed]
- Andrews, K.L. The whorlworm, Spodoptera frugiperda, in central America and neighboring areas. Fla. Entomol. 1980, 63, 456–467. [Google Scholar] [CrossRef]
- Buntin, G.D. A review of plant response to fall armyworm, Spodoptera frugiperda (J.E. Smith), injury in selected field and forage crops. Fla. Entomol. 1986, 69, 549–559. [Google Scholar] [CrossRef]
- Cruz, I.; Turpin, F.T. Effects of Spodoptera frugiperda on different growth stages of corn. Pesqui. Agropecu. Bras. 1982, 17, 355–359. [Google Scholar]
- De Almeida, S.R.; de Souza, A.R.W.; Vieira, S.M.J.; de Oliveira, H.G.; Holtz, A.M. Biology review, occurrence and control of Spodoptera frugiperda (Lepidoptera: Noctuidae) in corn in Brazil. Biosci. J. 2002, 18, 41–48. [Google Scholar]
- IPPC. Les dégâts causés par Spodoptera frugiperda. (The damage caused by Spodoptera frugiperda). In IPPC Official Pest Report; FAO: Rome, Italy, 2016; Available online: https://www.ippc.int/ (accessed on 15 April 2017).
- Rwomushana, I.; Bateman, M.; Beale, T.; Beseh, P.; Cameron, K.; Chiluba, M.; Clottey, V.; Davis, T.; Day, R.; Early, R.; et al. Fall Armyworm: Impacts and Implications for Africa; Evidence Note Update; CABI: Oxfordshire, UK, 2018. [Google Scholar]
- FAO. Fall Armyworm Outbreak, a Blow to Prospects of Recovery for Southern Africa. Available online: http://www.fao.org/africa/news/detail-news/en/c/469532/ (accessed on 12 November 2018).
- Cook, D.R.; Leonard, B.R.; Gore, J. Field and laboratory performance of novel insecticides against armyworms (Lepidoptera: Noctuidae). Fla. Entomol. 2004, 87, 433–439. [Google Scholar] [CrossRef]
- Agboyi, L.K.; Mensah, S.A.; Clottey, V.A.; Beseh, P.; Glikpo, R.; Rwomushana, I.; Day, R.; Kenis, M. Evidence of leaf consumption rate decrease in Fall armyworm, Spodoptera frugiperda, larvae parasitized by Coccygidium luteum. Insects 2019, 10, 410. [Google Scholar] [CrossRef]
- Devine, G.J.; Furlong, M.J. Insecticide use: Contexts and ecological successions. Agric. Hum. Values 2007, 24, 281–306. [Google Scholar] [CrossRef]
- Yu, S.J. Detection and biochemical characterization of insecticide resistance in fall armyworm (Lepidoptera: Noctuidae). J. Econ. Entomol. 1992, 85, 675–682. [Google Scholar] [CrossRef]
- Al-Sarar, A.; Hal, F.R.; Downer, R.A. Impact of spray application methodology on the development of resistance to cypermethrin and spinosad by fall armyworm Spodoptera frugiperda (J.E. Smith). Pest Manag. Sci. 2006, 62, 1023–1031. [Google Scholar] [CrossRef]
- Bateman, M.L.; Day, R.K.; Luke, B.; Edgington, S.; Kuhlmann, U.; Cock, M.J.W. Assessment of potential biopesticide options for managing fall armyworm (Spodoptera frugiperda) in Africa. J. Appl. Entomol. 2018, 1–15. [Google Scholar] [CrossRef]
- FAO. Integrated Management of the Fall Armyworm on Maize: A Guide for Farmer Field Schools in Africa. Available online: http://www.fao.org/3/I8665EN/i8665en.pdf (accessed on 2 February 2019).
- Rosell, G.; Quero, C.; Coll, J.; Guerrero, A. Biorational insecticides in pest management. J. Pestic. Sci. 2008, 33, 103–121. [Google Scholar] [CrossRef]
- Segnou, J.; Amougou, A.; Youmbi, E.; Njoya, J. Effect of chemical treatments on pests and diseases of pepper (Capsicum annuum L.). Greener J. Agric. Sci. 2013, 3, 12–20. [Google Scholar] [CrossRef]
- Duarte, J.P.; Redaelli, L.R.; Jahnke, S.M.; Trapp, S. Effect of Azadirachta indica (Sapindales: Meliaceae) oil on Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae and adults. Fla. Entomol. 2019, 102, 408–412. [Google Scholar] [CrossRef]
- Shafighi, Y.; Ziaee, M.; Ghosta, Y. Diatomaceous earth used against insect pests, applied alone or in combination with Metarhizium anisopliae and Beauveria bassiana. J. Plant Prot. Res. 2014, 54, 62–66. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Athanassiou, C.G.; Peteinatos, G.G.; Boukouvala, M.C.; Benelli, G. Insecticidal effect and impact of fitness of three diatomaceous earths on different maize hybrids for the eco-friendly control of the invasive stored-product pest Prostephanus truncatus (Horn). Environ. Sci. Pollut. Res. 2018, 25, 10407–10417. [Google Scholar] [CrossRef]
- Constanski, K.C.; Zorzetti, J.; Santoro, P.H.; Hoshino, A.T.; Neves, P.M.O.J. Inert powders alone or in combination with neem oil for controlling Spodoptera eridania and Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae. Ciênc. Agrár. 2016, 37, 1801–1810. [Google Scholar]
- Moore, W.S.; Profita, J.C.; Koehler, C.S. Soaps for home landscape insect control. Calif. Agric. 1979, 33, 13–14. [Google Scholar]
- Prasanna, B.M.; Huesing, J.E.; Eddy, R.; Peschke, V.M. Fall Armyworm in Africa: A Guide for Integrated Pest Management, 1st ed.; CIMMYT: CDMX, Mexico, 2018. [Google Scholar]
- Assefa, F.; Ayalew, D. Status and control measures of fall armyworm (Spodoptera frugiperda) infestations in maize fields in Ethiopia: A review. Cogent Food Agric. 2019, 5, 1641902. [Google Scholar] [CrossRef]
- Chimweta, M.; Nyakudya, I.W.; Jimu, L.; Mashingaidze, A.B. Fall armyworm [Spodoptera frugiperda (J.E. Smith)] damage in maize: Management options for flood-recession cropping smallholder farmers. Int. J. Pest Manag. 2019, 66, 142–154. [Google Scholar] [CrossRef]
- Harrison, R.D.; Thierfelder, C.; Baudron, F.; Chinwada, P.; Midega, C.; Schaffner, U.; Van Den Berg, J. Agro-ecological options for fall armyworm (Spodoptera frugiperda J.E. Smith) management: Providing low cost, smallholder friendly solutions to an invasive pest. J. Environ. Manag. 2019, 243, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Sisay, B.; Tefera, T.; Wakgari, M.; Ayalew, G.; Mendesil, E. The Efficacy of selected synthetic insecticides and botanicals against fall armyworm, Spodoptera frugiperda, in maize. Insects 2019, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Deryck, P.W. Laboratory rearing of the fall armyworm. Fla. Entomol. 1979, 62, 87–91. [Google Scholar]
- Cruz, I.; Lourdes, M.; Silva, D.; Foster, E. Efficiency of chemical pesticides to control Spodoptera frugiperda and validation of pheromone trap as a pest management tool in maize crop. Rev. Bras. Milho Sorgo 2010, 9, 107–122. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Lo, K.-C.; Yao, M.-C. Effects of household soap solutions on the mortality of the two-spotted spider mite, Tetranychus urticae Kock (Acari: Tetranychidae). Formos. Entomol. 2006, 26, 379–390. [Google Scholar]
- Williams, K.A.; Green, D.W.J.; Pascoe, D.; Gower, D.E. The acute toxicity of cadmium to different larval stages of Chrionomus riparius (Diptera: Chironomidae) and its ecological significance for pollution regulation. Oecologia 1986, 70, 362–366. [Google Scholar] [CrossRef]
- Akossou, A.Y.J.; Attakpa, E.Y.; Fonton, N.H.; Sinsin, B.; Bosma, R.H. Spatial and temporal analysis of maize (Zea mays) crop yields in Benin from 1987 to 2007. Agric. For. Meteorol. 2016, 220, 177–189. [Google Scholar] [CrossRef]
- Amin, M.R.; Tithi, D.A.; Azad, H.M.S.; Hossain, S.M.A. Evaluation of soap solution for management of cotton sucking pests at different locations of Bangladesh. J. Sci. Technol. 2008, 6, 1–5. [Google Scholar]
- Adéyè, A.T.; Sikirou, R.; Boukari, S.; Aboudou, M.; Amagnidé, G.Y.G.A.; Idrissou, B.S.; Idrissou-Touré, M.; Zocli, B. Protection of maize crop against Spodoptera frugiperda with insecticides PlantNeem, Lambdace 25 EC and Viper 46 EC and yield loss reduction in Benin. J. Rech. Sci. Univ. Lomé 2018, 20, 1–13. [Google Scholar]
- Mitchell, R.D.; Mott, D.W.; Dhammi, A.; Reisig, D.D.; Roe, R.M.; Stewart, D. Field evaluation of a new thrips control agent for cotton: A mechanical insecticide. In Proceedings of the 2018 Beltwide Cotton Conferences, San Antonio, TX, USA, 3–5 January 2018. [Google Scholar]
- Curkovic, T. Detergents and soaps as tools for IPM in agriculture. In Integrated Pest Management (IPM): Environmentally Sound Pest Management; Gill, H.K., Goyal, G., Eds.; IntechOpen: London, UK, 2016; pp. 156–189. [Google Scholar]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: New York, NY, USA, 1971; p. 333. [Google Scholar]
- Savi, M.K.; Mangamana, E.T.; Jean Marcel Deguenon, J.M.; Hounmenou, C.G.; Kakaï, R.G. Determination of lethal concentrations using an R software function integrating the Abbott correction. J. Agric. Sci. Technol. 2017, 7, 25–30. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-147. Available online: https://CRAN.R-project.org/package=nlme>/ (accessed on 15 May 2017).
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Machler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for Zero-inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- OANDA. Available online: https://www1.oanda.com/currency/converter/ (accessed on 16 May 2020).
- Stewart, D.A.; (Imerys Filtration Minerals, Inc., Roswell, GA, USA). Personal communication, 2020.
- Osborne, L.S. Is soap a viable method for controlling Tetranychus urticae on plants in the interior environment? Proc. Fla. State Hort. Soc. 1982, 95, 149–151. [Google Scholar]
- Osborne, L.S. Soap spray: An alternative to conventional acaricide for controlling the twospotted spider mite (Acari: Tetranychidae) in greenhouses. J. Econ. Entomol. 1984, 77, 734–737. [Google Scholar] [CrossRef]
- Ware, G.W. The Pesticide Book, 5th ed.; Thomson Publications: Fresno, CA, USA, 2000; p. 418. [Google Scholar]
- Szumlas, D.E. Behavioral responses and mortality in German cockroaches (Blattodea: Blatellidae) after exposure to dishwashing liquid. J. Econ. Entomol. 2002, 95, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Koehler, C.S.; Barclay, L.W.; Kretchun, T.M. Soaps as insecticides. Calif. Agric. 1983, 37, 11–12. [Google Scholar]
- Jaramillo-Barrios, C.I.; Varón-Devia, E.H.; Monje-Andrade, B. Economic injury level and action thresholds for Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) in maize crops. Rev. Fac. Nac. Agron. Medellín 2020, 73, 9065–9076. [Google Scholar] [CrossRef]
- Kuate, A.F.; Hanna, R.; Fotio, A.R.P.D.; Abang, A.F.; Nanga, S.N.; Ngatat, S.; Tindo, M.; Masso, C.; Rose Ndemah, R.; Suh, C.; et al. Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) in Cameroon: Case study on its distribution, damage, pesticide use, genetic differentiation and host plants. PLoS ONE 2019, 14, e0215749. [Google Scholar]
- FAO; PPD. Manual on Integrated Fall Armyworm Management. Available online: https://doi.org/10.4060/ca9688en (accessed on 10 December 2020).
- Mkenda, P.; Mwanauta, R.; Stevenson, P.C.; Ndakidemi, P.; Mtei, K.; Belmain, S.R. Extracts from field margin weeds provide economically viable and environmentally benign pest control compared to synthetic pesticides. PLoS ONE 2015, 10, e0143530. [Google Scholar] [CrossRef]
- Dougoud, J.; Toepfer, S.; Bateman, M.; Jenner, W.H. Efficacy of homemade botanical insecticides based on traditional knowledge. A review. Agron. Sustain. Dev. 2019, 39, 37. [Google Scholar] [CrossRef]
- Babendreier, D.; Agboyi, L.K.; Beseh, P.; Osae, M.; Nboyine, J.; Ofori, S.E.K.; Frimpong, J.O.; Clottey, V.A.; Kenis, M. The efficacy of alternative, environmentally friendly plant protection measures for control of Fall armyworm, Spodoptera frugiperda, in maize. Insects 2020, 11, 240. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.X.; Stansly, P.A. Insecticidal activity of surfactants and oils against silverleaf whitefly (Bemisia argentifolii) nymphs (Homoptera: Aleyrodidae) on collards and tomato. Pest Manag. Sci. 2000, 56, 861–866. [Google Scholar] [CrossRef]
- Isman, M.B. Neem and related natural products. In Biopesticides: Use and Delivery; Hall, F.R., Menn, J.J., Eds.; Humana Press: Totowa, NJ, USA, 1999; Volume 5, pp. 139–153. [Google Scholar]
- Erler, F.; Cetin, H.; Saribasak, H.; Serttas, A. Laboratory and field evaluations of some botanical pesticides against the cedar leaf moth, Acleris undulana. J. Pest Sci. 2010, 83, 265–272. [Google Scholar] [CrossRef]
- Phambala, K.; Tembo, Y.; Kasambala, T.; Kabambe, V.H.; Stevenson, P.C.; Belmain, S.R. Bioactivity of common pesticidal plants on Fall armyworm larvae (Spodoptera frugiperda). Plants 2020, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Showler, A.T.; Flores, N.; Caesar, R.M.; Mitchel, R.D.; De León, A.A.P. Lethal effects of a commercial diatomaceous earth dust product on Amblyomma americanum (Ixodida: Ixodidae) larvae and nymphs. J. Med. Entomol. 2020, 57, 1575–1581. [Google Scholar] [CrossRef]
- Deguenon, J.M.; Azondekon, R.; Agossa, F.R.; Padonou, G.G.; Anagonou, R.; Ahoga, J.; N’dombidje, B.; Akinro, B.; Stewart, D.A.; Wang, B.; et al. ImergardTMWP: A non-chemical alternative for an indoor residual spray, effective against pyrethroid-resistant Anopheles gambiae (s.l.) in Africa. Insects 2020, 11, 322. [Google Scholar] [CrossRef]
- Deguenon, J.M.; Riegel, C.; Cloherty-Duvernay, E.R.; Stewart, D.A.; Wang, B.; Gittins, D.; Tihomirov, L.; Apperson, C.S.; McCord, M.G.; Roe, R.M. New mosquitocide derived from volcanic rock. J. Med. Entomol. 2020. [Google Scholar] [CrossRef]
- Korunic, Z. Diatomaceous earths, a group of natural insecticides. J. Stored Prod. Res. 1998, 4, 87–97. [Google Scholar] [CrossRef]
- Arthur, F.H. Toxicity of diatomaceous earth to red flour beetles and confused flour beetles (Coleoptera: Tenebrionidae): Effects of temperature and relative humidity. J. Econ. Entomol. 2000, 93, 526–532. [Google Scholar] [CrossRef]
- Fields, P.; Korunic, Z. The effect of grain moisture content and temperature on the efficacy of diatomaceous earths from different geographical locations against stored-product beetles. J. Stored Prod. Res. 2000, 36, 1–13. [Google Scholar] [CrossRef]
- Subramanyam, B.; Roesli, R. Inert dusts. In Alternatives to Pesticides in Stored-Product IPM; Subramanyam, B., Hagstrum, D.W., Eds.; Kluwer Academic Publishers: Boston, MA, USA, 2000; pp. 321–380. [Google Scholar]
- Athanassiou, C.G.; Kavallieratos, N.G.; Tsaganou, F.C.; Vayias, B.J.; Dimizas, C.B.; Buchelos, C.T. Effect of grain type on the insecticidal efficacy of SilicoSec against Sitophilus oryzae (L.) (Coleoptera: Curculionidae). Crop. Prot. 2003, 22, 1141–1147. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Peteinatos, G.G.; Petrou, S.E.; Boukouvala, M.C.; Tomanovic, Z. Influence of temperature and humidity on insecticidal effect of three diatomaceous earth formulations against larger grain borer (Coleoptera: Bostrychidae). J. Econ. Entomol. 2007, 100, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Dhammi, A.; Roe, R.M.; Stewart, D. Novel mechanical pesticides for tobacco thrips, Frankliniela fusca, control in cotton. In Proceedings of the 2016 Beltwide Cotton Conferences, New Orleans, LA, USA, 5–7 January 2016. [Google Scholar]
- Glenn, D.M.; Puterka, G.J.; Drake, S.R.; Unruh, T.R.; Knight, A.L.; Baherle, P.; Prado, E.; Baugher, T.A. Particle film application influences apple leaf physiology, fruit yield, and fruit quality. J. Am. Soc. Hortic. Sci. 2001, 126, 175–181. [Google Scholar] [CrossRef]
- Lapointe, S.L.; Mckenzie, C.L.; Hall, D.G. Reduced oviposition by Diaprepes abbreviates (Coleoptera: Curculionidae) and growth enhancement of citrus by surround particle film. J. Econ. Entomol. 2006, 99, 109–116. [Google Scholar] [CrossRef]
- Epstein, E. Silicon. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 41–64. [Google Scholar] [CrossRef]
- Ma, J.F.; Mitani, N.; Nagao, S.; Konishi, S.; Tamai, K.; Iwashita, T.; Yano, M. Characterization of the silicon uptake and molecular mapping of the silicon transporter gene in rice. Plant Physiol. 2004, 136, 84–89. [Google Scholar] [CrossRef]
- Bezuidenhout, S.R.; Nunkumar, A. Chemical Control Options for Fall Armyworm in Maize. Research & Technology Bulletin (2016–2017). Available online: https://www.kzndard.gov.za (accessed on 3 June 2018).
- Comité National de Gestion des Pesticides (CNGP). Available online: https://zoomagro.com/wp-content/uploads/2020/03/Liste-actialis%C3%A9e-des-pesticides-autoris%C3%A9s_F%C3%A9vrier-2020-1.pdf (accessed on 15 April 2020).
- Houngbo, S.; Zannou, A.; Aoudji, A.; Sinzogan, A.; Sossou, C.H.; Sikirou, R.; Zossou, E.; Totin Vodounon, S.H.; Adomou, A.; Ahanchede, A. Farmers’ knowledge and management practices of fall armyworm, Spodoptera frugiperda J.E. Smith in Benin. Agriculture 2020, 10, 430. [Google Scholar] [CrossRef]
- Jansson, R.K.; Dybas, R.A. Avermectins: Biochemical mode of action, biological activity and agricultural importance. In Insecticides with Novel Modes of Action-Mechanisms and Application; Ishaava, I., Degheele, D., Eds.; Springer: Berlin, Germany, 1998; pp. 153–170. [Google Scholar]
- Deng, L.; Chen, L.; Guan, S.; Liu, J.; Liang, J.; Li, X.; Li, Z. Dissipation of emamectin benzoate residues in rice and rice-growing environments. Molecules 2020, 25, 483. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, P.; Zhang, F.; Li, Y.; Du, F.; Pan, C. Dissipation and residue behavior of emamectin benzoate on apple and cabbage field application. Ecotoxicol. Environ. Saf. 2012, 78, 260–264. [Google Scholar] [CrossRef]
- Niu, C.; Wang, C.; Wu, C.; Yang, J.; Yanan Wen, Y.; Meng, S.; Lin, X.; Pang, X.; An, L. Toxic effects of the emamectin benzoate exposure on cultured human bronchial epithelial (16HBE) cells. Environ. Pollut. 2020, 257, 113618. [Google Scholar] [CrossRef]
- Shivalingaswamy, T.M.; Kumar, A.; Satpathy, S.; Rai, A.B. Efficacy of emamectin benzoate in the management of vegetable pests. Prog. Hortic. 2008, 40, 193–197. [Google Scholar]
- Gacemi, A.; Guenaoui, Y. Efficacy of emamectin benzoate on Tuta absoluta Meyrick (Lepidoptera: Gelechiidae) infesting a protected tomato crop in Algeria. Acad. J. Entomol. 2012, 5, 37–40. [Google Scholar]
- Soglo, Y.Y.; Nonvide, G.A.N. Climate change perceptions and responsive strategies in Benin: The case of maize farmers. Clim. Chang. 2019, 155, 245–256. [Google Scholar] [CrossRef]
- Tovihoudji, P.G.; Akponikpè, P.B.I.; Agbossou, E.K.; Bielders, C.L. Variability in maize yield and profitability following hill-placement of reduced mineral fertilizer and manure rates under smallholder farm conditions in northern Benin. Field Crops Res. 2019, 230, 139–150. [Google Scholar] [CrossRef]
- Abadassi, J. Caractérisation de quelques variétés améliorées de maïs cultivées au Bénin. Bull. Rech. Agron. 2001, 34, 1–6. [Google Scholar]


| Products a | Dose/ha | Surfactant (Palmida Soap) kg | Volume of Application (L) |
|---|---|---|---|
| Dezone 1 | 7.5 kg | 0.15 | 300 |
| Dezone 2 | 15 kg | 0.15 | 300 |
| Palmida soap | 1.5 kg | - | 300 |
| Emacot 19 EC | 0.6 L | - | 300 |
| PlantNeem | 4.5 L | 0.6 | 300 |
| N° | Items | Units | Unit Price (US $) a | Quantity/ha Per Crop Cycle |
|---|---|---|---|---|
| 1 | Maize seeds (Synée 2000) | Kg | 1.6849 | 20 |
| 2 | Water | L | 0.0016 (0) | Variable |
| 3 | Fertilizer NPK | Bag of 50 kg | 20.2190 (0) | 4 |
| 4 | Fertilizer Urea | Bag of 50 kg | 20.2190 (0) | 2 |
| 5 | Knapsack sprayer (16 L) b | Unit | 50.5475 | 2 |
| 6 | Protective clothing | Unit | 8.4245 | 2 |
| 7 | Emacot 19 EC | L | 8.4245 | 1.2 |
| 8 | Palmida soap | Bar | 0.2527 | Variable |
| 9 | PlantNeem | L | 5.3917 | 18 |
| 10 | Dezone | Kg | 1.8888 | 30 |
| 11 | Labor for weeding | Man day | 5.0547 (3.3698) | 8 (16) |
| 12 | Labor for fertilizer application | Man day | 2.5273 (0) | 12 (0) |
| 13 | Labor for spraying | Man day | 8.4245 (3.3698) | Variable |
| 14 | Labor for harvesting | Man day | 2.5273 (3.3698) | 8 (12) |
| Soap/Detergent | N a | Slope (SE b) | LC50 c (95% CL d) | χ2 e |
|---|---|---|---|---|
| Klin | 700 | 1.84 (0.43) | 0.462 (0.36–0.60) | 2.22 |
| Koto | 700 | 1.84 (0.43) | 0.443 (0.39–0.58) | 3.19 |
| Palmida | 700 | 1.74 (0.44) | 0.373 (0.27–0.51) | 3.33 |
| Sites | Treatments | Growth Parameters a | ||
|---|---|---|---|---|
| Plant Height (m) | Stem Thickness (cm) | Leaf Number | ||
| Adjohoun | Dezone 1 | 1.63 ± 0.04 ab | 3.45 ± 0.17 a | 11.32 ± 0.19 a |
| Dezone 2 | 1.58 ± 0.04 ab | 3.25 ± 0.17 ab | 11.07 ± 0.23 a | |
| Emacot 19 EC | 1.71 ± 0.03 a | 2.87 ± 0.07 bc | 11.42 ± 0.19 a | |
| PlantNeem | 1.51 ± 0.05 b | 3.17± 0.14 ab | 10.73 ± 0.23 a | |
| Palmida soap | 1.52 ± 0.03 b | 2.55 ± 0.06 cd | 11.30 ± 0.17 a | |
| Control | 1.62 ± 0.04 ab | 2.43 ± 0.07 d | 11.25 ± 0.18 a | |
| N’Dali | Dezone 1 | 1.89 ± 0.02 a | 2.81 ± 0.03 ab | 11.38 ± 0.15 a |
| Dezone 2 | 1.89 ± 0.02 a | 2.72 ± 0.04 a | 11.09 ± 0.15 a | |
| Emacot 19 EC | 1.75 ± 0.02 b | 2.65 ± 0.04 b | 10.73 ± 0.14 a | |
| PlantNeem | 1.75 ± 0.02 b | 2.78 ± 0.04 a | 10.94 ± 0.15 a | |
| Palmida soap | 1.71 ± 0.02 b | 2.80 ± 0.04 a | 10.85 ± 0.15 a | |
| Control | 1.62 ± 0.02 c | 2.45 ± 0.04 c | 9.99 ± 0.15 a | |
| Sites | Treatments | Yield (kg·ha−1) a | Percentage Reduction in the Grain Yield Loss (%) | |
|---|---|---|---|---|
| Maize Cob | Maize Grain | |||
| Adjohoun | Negative Control | 3538 ± 322 b | 2060 ± 211 b | - |
| PlantNeem | 4840 ± 1030 ab | 3618 ± 826 ab | 43 | |
| Palmida soap | 4975 ± 608 ab | 3830 ± 543 ab | 46 | |
| Dezone 2 | 5298 ± 953 ab | 3968 ± 924 ab | 48 | |
| Dezone 1 | 5928 ± 572 ab | 4408 ± 357 ab | 53 | |
| Emacot 19 EC | 7143 ± 533 a | 5308 ± 565 a | 61 | |
| N’Dali | Negative control | 5718 ± 901 b | 4612 ± 766 b | - |
| PlantNeem | 7163 ± 724 ab | 5804 ± 657 ab | 21 | |
| Palmida soap | 8056 ± 859 a | 6577 ± 754 ab | 30 | |
| Emacot 19 EC | 8312 ± 1065 a | 6694 ± 1016 a | 31 | |
| Dezone 2 | 8073 ± 591 a | 6712 ± 520 a | 31 | |
| Dezone 1 | 9003 ± 868 a | 7387 ± 735 a | 38 | |
| District | Treatments | Grain Yields (Kg/ha) | Average Revenue (US $ per ha) a | Total Cost (US $ per ha) | Profit (US $ per ha) | Net Gain in Comparison to the Negative Control (%) b |
|---|---|---|---|---|---|---|
| Negative control | 2060 | 520.64 | 128.05 | 392.59 | - | |
| PlantNeem | 3618 | 914.41 | 413.14 | 501.27 | 28 | |
| Adjohoun | Palmida soap | 3830 | 967.99 | 325.18 | 642.81 | 64 |
| Dezone | 4408 | 1114.08 | 368.20 | 745.88 | 90 | |
| Emacot 19 EC | 5308 | 1339.71 | 269.54 | 1043.16 | 166 | |
| Negative control | 4612 | 1165.62 | 245.99 | 919.62 | - | |
| PlantNeem | 5804 | 1466.89 | 519.59 | 947.29 | 3 | |
| N’Dali | Palmida soap | 6577 | 1662.25 | 431.67 | 1230.58 | 34 |
| Emacot 19 EC | 6694 | 1691.82 | 408.75 | 1283.06 | 40 | |
| Dezone | 7387 | 1866.97 | 474.69 | 1392.28 | 51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aniwanou, C.T.S.; Sinzogan, A.A.C.; Deguenon, J.M.; Sikirou, R.; Stewart, D.A.; Ahanchede, A. Bio-Efficacy of Diatomaceous Earth, Household Soaps, and Neem Oil against Spodoptera frugiperda (Lepidoptera: Noctuidae) Larvae in Benin. Insects 2021, 12, 18. https://doi.org/10.3390/insects12010018
Aniwanou CTS, Sinzogan AAC, Deguenon JM, Sikirou R, Stewart DA, Ahanchede A. Bio-Efficacy of Diatomaceous Earth, Household Soaps, and Neem Oil against Spodoptera frugiperda (Lepidoptera: Noctuidae) Larvae in Benin. Insects. 2021; 12(1):18. https://doi.org/10.3390/insects12010018
Chicago/Turabian StyleAniwanou, Crépin T. S., Antonio A. C. Sinzogan, Jean M. Deguenon, Rachidatou Sikirou, David A. Stewart, and Adam Ahanchede. 2021. "Bio-Efficacy of Diatomaceous Earth, Household Soaps, and Neem Oil against Spodoptera frugiperda (Lepidoptera: Noctuidae) Larvae in Benin" Insects 12, no. 1: 18. https://doi.org/10.3390/insects12010018
APA StyleAniwanou, C. T. S., Sinzogan, A. A. C., Deguenon, J. M., Sikirou, R., Stewart, D. A., & Ahanchede, A. (2021). Bio-Efficacy of Diatomaceous Earth, Household Soaps, and Neem Oil against Spodoptera frugiperda (Lepidoptera: Noctuidae) Larvae in Benin. Insects, 12(1), 18. https://doi.org/10.3390/insects12010018




